Abstract
Methods : This multicentric retrospective study aimed to evaluate the efficacy and the safety of the combination of BBV in patients with non-cutaneous R/R PTCL among 21 LYSA centers in France and Belgium.
The primary objective was to evaluate the best overall response rate (ORR) (complete response (CR) and partial response (PR)). Secondary objectives were progression free survival (PFS), overall survival (OS), duration of response (DoR), impact of transplantation on outcome, and safety. Patients treated between January 2013 and October 2020 were reviewed and all the data were collected through an electronic questionnaire sent to all the physicians.
Results : Eighty two patients with R/R PTCL (40 angioimmunoblastic lymphoma (AITL), 2 T-cell lymphoma with TFH phenotype ,13 PTCL not otherwise specified (PTCL NOS), 5 Alk+ anaplastic large cell lymphoma (ALCL), 17 Alk- ALCL, , 1 Extranodal NK-/T-cell lymphoma, 3 Enteropathy-associated T-cell lymphoma (EATL), 1 subcutaneous panniculitis) were included. Median age at beginning of BBV was 60 years, most of patients were male (61%), had advanced stage (88%) and an IPI ≥ 2 (79%). Half of patients were refractory to their last treatment. Median number of prior regimens was 1 (range 1 to 6).
The best ORR was 71%, with 51% of patients in CR. In multivariable analysis, only the relapse status after the last regimen (relapse vs refractory) was associated with ORR, relapsed patients having a better ORR (83% vs 57% in refractory patients, p=.014, OR=3.70 (95%CI:1.3-10.5)). Median DoR was 15.4 months in patients with CR but differed significantly whether patients were transplanted or not (Not reached vs 8.4 months, p=.0055).
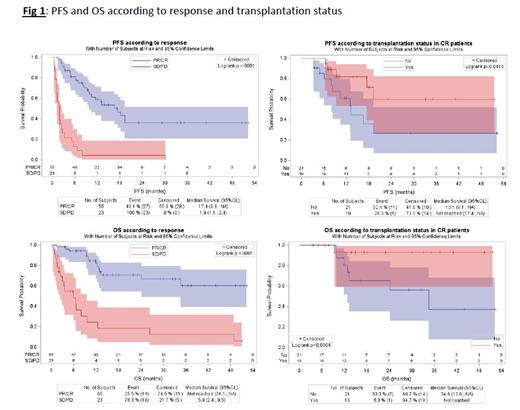
Twenty-two patients (30% of patients ≤ 70 years of age) were transplanted (6 autologous and 16 allogenic). With a median follow-up of 9 months, the median PFS and OS were 8.3 and 26.3 months respectively.
In multivariable analysis, only 2 factors had a significant impact on PFS and OS: best response (CR/PR vs SD/PD with a median PFS of 17.4 vs 1.9 months, p<.0001, and a median OS Not Reached vs 5,9 months, p<.0001) and transplantation (for patients in CR, median PFS was Not Reached in transplanted patients vs 13.1 months; p=.0410, and median OS was Not Reached vs 34, 6 months; p=.0304) (Fig1). Histological subgroups was also significantly associated with PFS (p=.012) but not with OS (p=.26) in multivariable analysis. Patients with PTCL NOS/Other subtypes had worse PFS than patients with TFH subtypes (HR=2.89 (95%CI: 1.4-5.8), p=.0029). Interestingly the CD30 status (positive vs negative) had no impact on ORR or survival.
Fifty-nine percent of patients experienced a grade 3 to 4 adverse event which was mainly hematologic toxicity. Treatment had to be stopped in 11% of patients.
Conclusion:
To the best of our knowledge, this is the first study reporting on the combination of BBV in the treatment of R/R PTCL in such a large cohort.
The results are very encouraging with a high response rate, long DoR in responding patients and a very good outcome. Furthermore, patients in CR who are eligible for transplant have the best outcome, making this combination a good candidate as salvage therapy before transplant consolidation in these high-risk lymphomas with limited treatment options.
Bouabdallah: Kite/Gilead: Consultancy, Honoraria; Takeda: Consultancy, Honoraria; Roche: Consultancy, Honoraria. Herbaux: Takeda: Honoraria, Research Funding; Janssen: Honoraria; Roche: Honoraria; Abbvie: Honoraria, Research Funding. Brice: MSD: Research Funding; Amgen: Other: Travel/accommodations/expenses; Roche: Other: Travel/accommodations/expenses; Takeda: Research Funding. Sibon: Abbvie: Consultancy; Janssen: Consultancy; Roche: Consultancy; iQone: Consultancy; Takeda: Consultancy. Laribi: AstraZeneca: Other: Personal Fees; AbbVie: Other: Personal Fees, Research Funding; IQONE: Other: Personal Fees; Astellas Phama, Inc.: Other: Personal Fees; BeiGene: Other: Personal Fees; Takeda: Other: Personal Fees, Research Funding; Novartis: Other: Personal Fees, Research Funding; Le Mans Hospital: Research Funding; Jansen: Research Funding. Damaj: roche: Consultancy, Honoraria; takeda: Consultancy, Honoraria.
Brentuximab Vedotin and Bendamustine

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal